Thomson atomic model:
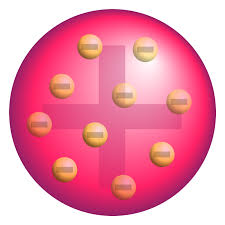
Thomson atomic model/watermelon model of atom says that An atom is similar to a watermelon in which electrons are like watermelon seeds and the flesh is positive sphere. Thus electrons are present in the positive sphere of atom.
The over all atom is neutral because positive and negative charges are equal in number.
J.J. Thomson’s proposed watermelon model of atom, originating in 1904, was referred to as the “plum pudding model” and suggested that atoms are made up of electrons within a sphere of positive charge; much like raisins in a pudding. This idea was based on cathode ray tube experiments which revealed that the negatively charged cathode rays (now known to be electrons) were deflected by electromagnetic fields. The positive charge was meant to balance out the negative charge of the electrons and make the atom neutral.
However, this model failed to properly explain observations such as element stability and spectral lines, leading to its dismissal in favor of other atomic models such as those proposed by Ernest Rutherford and Niels Bohr. Despite being proven wrong, Thomson’s atomic model marked an important milestone within atomic theory as it brought about the discovery of the electron and initiated our understanding towards atomic structure.
In 1904, English physicist J.J. Thomson put forward the Atomic Model of Thomson, better known as the “plum pudding” model. According to this model, Thomson suggested that atoms are composed of negatively-charged electrons randomly scattered in a sphere of positive charge, similar to raisins in a pudding. This idea was formed after he conducted experiments with cathode ray tubes, which showed that the rays were negatively charged and could be deflected by electric and magnetic fields. To make sure the atom was neutral overall, Thomson proposed that these electrons were embedded in the sphere of positive charge. Eventually, though his model failed to explain certain observations such as stable elements and spectral lines, it helped develop our current understanding of atomic structures because it marked the discovery of electrons and began an exploration into atomic behavior.
Reas about Niels Bohr discovery
Buy an atomic model kit here.


