PPT 4.4 Intermolecular forces has the following keywords: London (dispersion) forces, instantaneous dipole, induced dipole, van der Waals’ forces, dipole-dipole interactions, hydrogen bonding, intramolecular, intermolecular,tetrahedral
This PPT 4.4 Intermolecular forces aligns with the IB Diploma Chemistry Guide. It covers the past paper questions on this subtopic.
Objectives: This PPT enables the students to apply their learning of structure and chemical formula to deduce the types of intermolecular force present in chemical species. To explain the physical properties of covalent compounds like volatility, electrical conductivity and solubility in terms of their structure and intermolecular and intramolecular forces.
Guidance: The students often confuse about the use of the term London forces and Van der Waals’. You should use the term London forces for instantaneous and induced dipoles between any non polar atoms or groups of atoms. The term “van der Waals” is applicable for dipole–dipole, dipole-induced dipole and London forces together.
4.4 Intermolecular forces
Intermolecular forces exists between the molecules to held them together. These forces include the London forces, Dipole-dipole, Vander Waals’ and Hydrogen bonding.
The strength of intermolecular forces
London forces<Dipole-dipole< Hydrogen bonding
The non-polar species have London forces. These are weak forces of attraction. The strength increases by increasing molar mass.
Example: Noble gases and non-polar molecules like methane
London forces exists due to creation of temporary or instantaneous dipole.
It happens due to uneven distribution of electrons around nucleus in valency shell.
Dipole Dipole interaction
Polar molecules become a dipole due to difference in electronegativity. Thus polar molecules or dipoles attracts each other. Such forces of attraction is stronger than London forces.
Example: H2O, NO2, HCl
Vander Waals’ forces
The weak forces of attraction including London forces, Dipole-dipole and induced dipole are commonly known as Vander Waals’ forces.
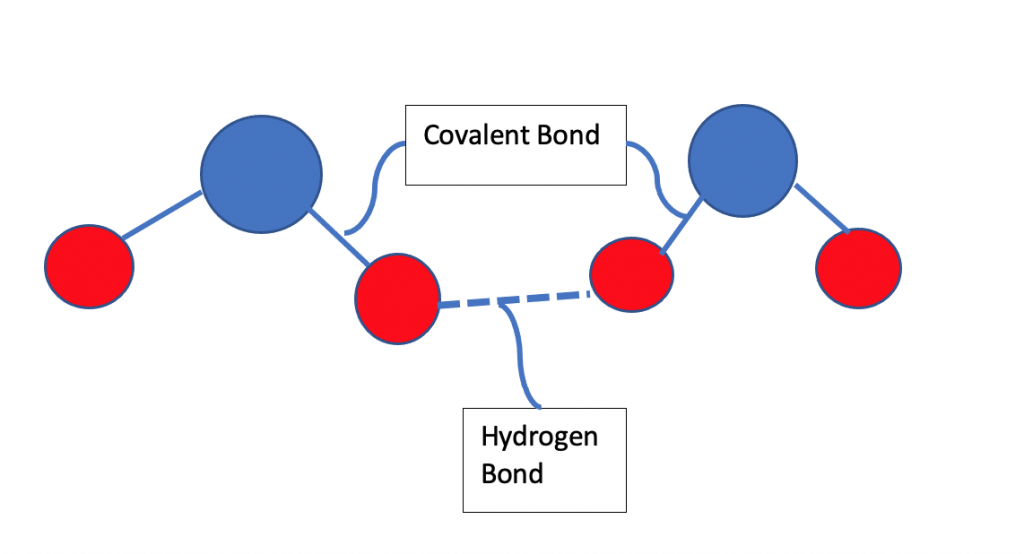
Hydrogen Bonding
The electronegative atom(FON) pulls the shared pair of electron towards itself from Hydrogen and becomes partially negative resulting hydrogen being partially positive.
Partial positive Hydrogen atom of one molecule gets attracted towards partial negative FON(Fluorine, Oxygen or Nitrogen) of another molecule. This force of attraction is strongest among all intermolecular forces.
Hydrogen bonded substances have higher BP and MP.
Example: H2O has high BP than H2S similarly HF has High BP than HCl
H2O has high BP which is due to its greater ability to have Hydrogen bonding. In solid state(ice) it can expand that is why ice can float over liquid H2O. Ice has H2O molecules in tetrahedral arrangement.H2O has highest density at 40 C.
You can look for chemistry IA templet here.
You can post your educational articles here on online educational magazine.
