PPT 4.2 Covalent bonding has the following keywords: Octet rule, duplet rule, Lewis diagram, covalent bond, multiple bond, bond strength, bond length, electronegativity polarity (polar bond)
This PPT 4.2 Covalent bonding aligns with the IB Diploma Chemistry Guide. It covers the past paper questions on this subtopic.
Objectives: This PPT enables the students to apply their learning to deduce the polar nature of a covalent bond from electronegativity values.
Guidance: Students should practice drawing the diagrams. Students should show the Bond polarity either with partial charges, dipoles or vectors.
The data booklet in section 8 has electronegativity values.
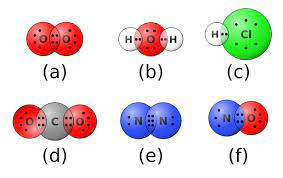
Wikimedia Image
The atoms try to become stable by acquiring the electronic structure of nearest noble gas. Except Helium all the noble gases have eight electrons in their outer most shell so atoms try to get eight electrons in the outermost shell. This is called Octet rule.
Atoms become stable by :
- Lose of outermost electrons( Metals become stable by lose of outermost electrons)
- Gain of electrons( Non-Metals become stable by gain of electrons) or
- Sharing of electrons(Non-Metals become stable by sharing of electrons)
The atoms near to He try to acquire 2 electrons in their outermost shell to become stable. It is called duplet rule.
The covalent bond is the electrostatic attraction between the bonded atom and the positively charged nucleus of opposite atom.
Atoms short of 1 electron to achieve noble gas configuration share 1 electron pair resulting single covalent bond.
Example: H2 Molecule, HCl Molecule
Lewis diagram to represent the bond
You need to show all the outermost electrons of the bonded atoms. You can represent the outermost electrons by dot and cross or by lines/dash.
Atoms short of 2 electrons to achieve noble gas configuration share 2 electron pair resulting double covalent bond.
You can look for chemistry IA templet here.
You can post your educational articles here on online educational magazine.
