PPT 2.2 Electron configuration has the following keywords:
Electron Configuration, Quantum, Uncertainty, Orbitals, Momentum, Probability, Wave, Aufbau, Node
This PPT 2.2 Electron configuration aligns with the IB Diploma Chemistry Guide. It covers the past paper questions on this subtopic.
Objectives: This PPT enables the students to apply their learning to differentiate between the shapes of atomic orbitals of s and p sub shells. The students can use Hund’s rule, Pauli exclusion principle and Aufbau rule to write the electronic configuration of atoms.
Though IB does not ask to learn quantum numbers it helps to understand the whole concept. Students can relate the existence of a particular sub-shell in a shell with quantum numbers. Students can differentiate between different sets of the electromagnetic spectrum. They can differentiate between absorption and emission spectrum. They can differentiate between line and continuous spectrum.
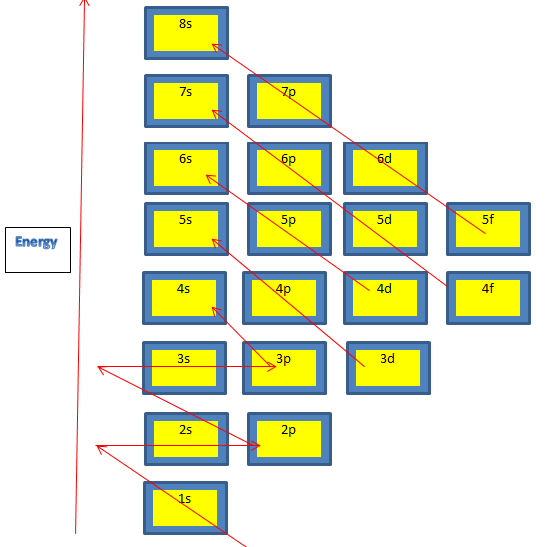
Guidance: You can find the electromagnetic spectrum with wavelengths in the data booklet in section 3. Students need not to know the names of Lyman, Balmer etc series in the hydrogen spectrum. Students should learn condensed, full as well as orbital diagrams of electronic configuration.
Students should know the Exceptional cases of Cu and Cr where 3d4 changes to 3d5 due to the symmetrical energy aspect.
Quantum mechanical model of Atom: It is based on Heisenberg’s uncertainty principle and Schrodinger’s equation. This model helps to understand the electronic structure of an atom more precisely. It explains the address of an electron inside an atom in the form of quantum numbers.
Principle Quantum Number:
It is denoted by “n”. It tells us about the energy and the distance of an electron from the nucleus.
So far we have 7 periods in periodic table hence 7 energy levels can be understood. Each energy level is related to each period.
These shells can also be designated as K L M N O P Q etc.
Azimuthal Quantum Number:
It is denoted by “l”. It’s value depends on Principle Quantum Number n. It tells us about sublevel of a shell that is subshell.
For each value of n, l= 0 to n-1.
So
n=1 means l=0 means “s” subshell
n=2means l=0, 1 means “s” and “p” subshells
n=3 means l=0, 1,2 means “s”, “p” and “d” subshells
n=4 means l=0, 1,2,3 means “s”, “p” , “d” and “f” subshells
n=5 means l=0, 1,2,3,4 means “s”, “p” , “d”, “f” and “g” subshells and so on…….
Magnetic Quantum Number:
It is denoted by “m”. It tells us about the orbitals. Each value of l corresponds to one orbital.
For each value of “l” m= -l to +l on number line.
So l=0 means m=0 means only one value means one orbital.
S subshell has only one orbital.
Spin Quantum Number:
It is denoted by “s” and has two values for each value of m,
+1/2(Clockwise spin) and -1/2(anticlockwise spin) which means two elctrons.
You can look for chemistry IA templet here.
You can post your educational articles here on online educational magazine.
