PPT 3.2 Periodic trends has the following keywords: Isoelectronic,Ionization,Electron Affinity,Enthalpy,Electronegativity,Pauling Scale,Amphoteric
This PPT 3.2 Periodic trends aligns with the IB Diploma Chemistry Guide. It covers the past paper questions on this subtopic.
Objectives: This PPT enables the students to apply their learning to :Predict and explain the properties of an element by looking its position in the periodic table. Discuss the properties of elements in a particular group for example group 1 of alkali metals and group 7 of halogens. Write equations for the reactions of sodium oxide, Magnesium oxide, Phosphorus oxide, nitrogen oxides and sulfur oxides with water.
Positive Ions are always smaller in size than the parent atoms due to increased nuclear attraction.
Same number of protons but less electron increases the nuclear attraction and the size becomes smaller.
Metals loose valence electrons to become positive ions( cations) because cations have more protons than electrons. Na atom has 11 p and 11 e but after lose of 1 e it left with 10 e but 11 p so 1 p is extra means 1 positive charge is extra so it becomes Na.+
Na – 1e—>Na+
2,8,1 2,8
3 shells 2 shells
Negative ions are always bigger in size than the parent atoms due to decreased nuclear attraction.
Same number of protons but more electron decreases the nuclear attraction and the size becomes bigger.
Non-Metals gain electrons to become negative ions( anions) because anions have less protons than electrons. Cl atom has 17 p and 17 e but after gain of 1 e it has with 18 e but 17 p so 1 p is less means 1 positive charge is less so it becomes Cl– .
Cl +1e —> Cl–
2,8,7 2,8,8
3 shells 3 shells
Isoelectronic
The ions with the same number of electrons.
The positive ions Na+,Mg2+,Al3+ etc have same number of electrons so these are isoelectronic.
The negaitive ions Cl-,S2-,P3- etc have same number of electrons so these are isoelectronic.
The isolectronic ions across the period decreases in size.
It is the amount of energy required to remove one mole of electron from one mole of gaseous atom. It is measured in Kj/mol.
M(g) – e —>M+(g)
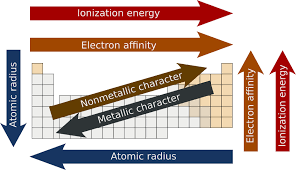
The Ionization energy always decreases down the group.
The Ionization energy generally increases across the period.
Exceptional example: Be has higher IE than B in second period because B= 1s2 2s2 2p1 while Be= 1s2 2s2 so it is easier to remove an electron from B than Be.
Electron Affinity or Electron gain enthalpy
It is the enthalpy change which takes place when one mole of electons are added to one mole of gaseous atom. The overall enthalpy change can be positive or negative.
X(g) + e —> X-(g)
The electron gain enthalpy increases across the period and decreases down the group.
Exceptional example: F has lower electron gain enthalpy than Cl.
Electronegativity
It is the tendency to pull the shared pair of electron in a covalent bond. It is represented by Pauling scale.
The electronegativity increases across the period.
The electronegativity decreases down the group.The three most electronegative elements are FON.
Oxides of metals and nonmetals
Across the period the elements are ranging from metal to nonmetal.
Example : In period 3, Na, Mg form metallic but basic oxides, Al forms metallic but amphoteric oxide while Si, P, S and Cl form non metallic but acidic oxides.
Example 4Na + O2 —> 2Na2O
2Mg + O2 —>2MgO
4Al + O2—>2Al2O3
2Si + 2O2 —> 2SiO2
4P + 5O2 —>2P2O5
You can look for chemistry IA templet here.
You can post your educational articles here on online educational magazine.
