4.1 Ionic bonding and structure has the following keywords: Electrovalent, Cation, Anion, Ionic Bond, Electron Affinity, Lattice Enthalpy, Polyatomic Ion,Electrostatic,Crystalline
This PPT 4.1 Ionic bonding and structure aligns with the IB Diploma Chemistry Guide. It covers the past paper questions on this subtopic.
Objectives: This PPT enables the students to apply their learning to deduce the formula and name of an ionic compound from its constituent ions. They can apply the understanding to use the polyatomic ions in writing the formula and name. The students can discuss the physical properties of ionic compounds like volatility, electrical conductivity and solubility.
Guidance: Students should know the polyatomic ions like ammonium ion, Phosphate ion, hydroxide ion, nitrate ion, hydrogen carbonate and carbonate, sulphate ion and ethanoate ion.
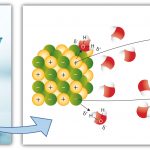
Atoms loose or gain electrons to achieve the nearest noble gas electronic configuration.
The electrostatic force of attraction between two or more oppositely charged ions is called ionic bond.
Example: The electrostatic force of attraction between Sodium ions (Na+)
and chloride ions( Cl–) forms an ionic bond.
Positive and negative ions always remain attracted by each other in a network called as lattice.
In solidum chloride, each Sodium ion (Na+) is attracted by six chloride ions( Cl–) and vice versa.
Metals loose valence electrons to become positive ions( cations) because cations have more protons than electrons. Apart from metals another positive ion is polyatomic which is Ammonium ion NH4+
Example: Na atom has 11 p and 11 e but after lose of 1 e it left with 10 e but 11 p so 1 p is extra means 1 positive charge is extra so it becomes Na.+
Na – 1e —>Na+
2,8,1 2,8
Non-Metals gain electrons to become negative ions( anions) because anions have less protons than electrons.
You can look for chemistry IA templet here.
You can post your educational articles here on online educational magazine.
