Rutherford’s nuclear Model of Atom is based on the gold foil experiment with alpha particles.
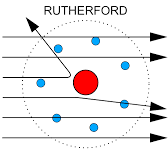
A gold foil was selected because it is very thin. The foil was bombarded by alpha particles which are Helium Nucleus.
Most alpha particles pass through the gold foil because most of the atom is hollow.
Some Alpha particles are repelled by the nucleus because the nucleus is also positive and Alpha particles are positive Helium Nucleus.
Very few Alpha particles were bounced back because the nucleus is solid.
He proposed the following Atomic model:
Atom is mostly emplty or hollow except the dense center which is nucleus.
Nucleus has protons( positively charged particles) and Neutrons(Neutral particles).
Electrons ( Negatively charged particles) revolve around the nucleus in fixed circular orbits.
In contrast to the existing “plum pudding” model of that time which suggested an even distribution of electrons throughout the atom, Rutherford’s model had a comparatively tiny nucleus at its centre that contains protons and neutrons, with an electron cloud surrounding it where the electrons are located. This atomic model is now called Rutherford nuclear model, and it was confirmed when he conducted his gold foil experiment, in which positively charged alpha particles were fired at a thin sheet of gold foil — most passed through unobstructed as though there were nothing there but some were deflected at sharp angles when they collided with something dense and positively charged. Even today, this is still one of the foundations for modern atomic physics, although it lacks much detail about how electrons actually work within atoms.

The Contributions of Niels Bohr to Quantum Mechanics


