2.2 The Electron configuration
Quantum mechanical model of Atom:
It is based on Heisenberg’s uncertainty principle and Schrodinger’s equation. This model helps to understand the electronic structure of an atom more precisely. It explains the address of an electron inside an atom in the form of quantum numbers.
Principle Quantum Number:
It is denoted by “n”. It tells us about the energy and the distance of an electron from the nucleus.
So far we have 7 periods in periodic table hence 7 energy levels can be understood. Each energy level is related to each period.
These shells can also be designated as K L M N O P Q etc.
Azimuthal Quantum Number:
It is denoted by “l”. It’s value depends on Principle Quantum Number n. It tells us about sublevel of a shell that is subshell.
For each value of n, l= 0 to n-1.
So
n=1 means l=0 means “s” subshell
n=2means l=0, 1 means “s” and “p” subshells
n=3 means l=0, 1,2 means “s”, “p” and “d” subshells
n=4 means l=0, 1,2,3 means “s”, “p” , “d” and “f” subshells
n=5 means l=0, 1,2,3,4 means “s”, “p” , “d”, “f” and “g” subshells and so on…….
Magnetic Quantum Number:
It is denoted by “m”. It tells us about the orbitals. Each value of l corresponds to one orbital.
For each value of “l” m= -l to +l on number line.
So l=0 means m=0 means only one value means one orbital.
S subshell has only one orbital.
Spin Quantum Number:
It is denoted by “s” and has two values for each value of m,
+1/2(Clockwise spin) and -1/2(anticlockwise spin) which means two elctrons.
| Principle(n) | Azimuthal(l=0 to n-1) | Subshell | Magnetic(m=-l,0,+l) | e | |
| 1 | 0 | s | 0 | 2 | |
| 2 | 0,1 | s,p | 0,-1,0,+1 | 8 | |
| 3 | 0,1,2 | s,p,d | 0,-1,0,1,-2,-1,0,1,2 | 18 | |
| 4 | 0,1,2,3 | s,p,d,f | 0,-1,0,1,-2,-1,0,1,2,-3,-2,-1,0,1,2,3 | 32 |
Thus we seen that p subshell start with n=2, d subshell starts with n=3 and f subshell starts with n=4.
Heisenberg uncertainty principle, it is impossible to determine momentum and position of an electron in an atom simultaneously.
If position is to determine its momentum will change and vice versa because light(energy) is to be dropped on an electron to track it, which will change the position of the electron.
Momentum (p) = mass(m) x Velocity(v)
Hence if its not possible to determine the position of an electron , how can we say that electron revolve around the nucleus in discrete orbits. We can only find the probability of electron in some space, called orbital.
What is Schrodinger wave equation? :
Erwin Schrodinger gave a mathematical equation “Schrodinger wave equation”for the wave nature of electron Ĥψ = E ψ where H is Hamiltonian operator and E is total energy operator.
Solution of this equation using super computer gives numerical solutions, which we call as quantum numbers.
Hund’s Rule of Maximum Multiplicity
Hund’s Rule of Maximum Multiplicity suggests that Pairing of electrons does not take place until all the orbitals of a sub-shell are singly filled.
Pauli’s Exclusion principle for electronic configuration
Pauli’s Exclusion principle for electronic configuration suggests that No Two electrons can have same set of four quantum numbers. It means that an orbital can only have two electrons. This principle is used to write the orbital diagram of atoms along with Hund’s Rule of maximum multiplicity and Aufbau rule.
Aufbau Principle and filling of electrons in orbitals and sub shells
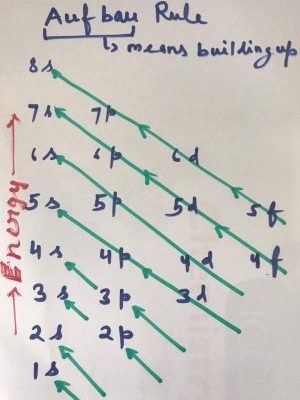
Aufbau Principle for electronic configuration is not on the name of any scientist as usually happened in sciences. Aufbau means building up.
Electrons are build up in orbitals of different subshells of different energy levels on the basis of energy.
Electrons first fill lower energy level and then move to next higher level.

s orbitals
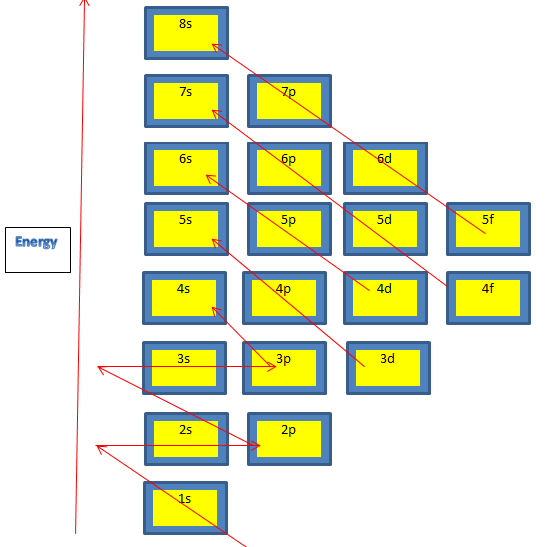
So electrons start filling from 1s orbital on wards in the following sequence:
1s,2s,2p , 3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p,8s
Simply it can be learned as ssp sps dps dps fdps fdps
| Atomic Number | Element | Electronic Configuration |
| 1 | Hydrogen | Is1 |
| 2 | Helium | Is2 |
| 3 | Lithium | 1s2 2s1 |
| 4 | Beryllium | 1s2 2s2 |
| 5 | Boron | 1s2 2s2 2p1 |
| 6 | Carbon | 1s2 2s2 2p2 |
| 7 | Nitrogen | 1s2 2s2 2p3 |
| 8 | Oxygen | 1s2 2s2 2p4 |
| 9 | Fluorine | 1s2 2s2 2p5 |
| 10 | Neon | 1s2 2s2 2p6 |
| 11 | Sodium | 1s22s22p63s1 |
| 12 | Magnesium | 1s22s22p63s2 |
| 13 | Aluminium | 1s22s22p63s23p1 |
| 14 | Silicon | 1s22s22p63s23p2 |
| 15 | Phosphorus | 1s22s22p63s23p3 |
| 16 | Sulphur | 1s22s22p63s23p4 |
| 17 | Chlorine | 1s22s22p63s23p5 |
| 18 | Argon | 1s22s22p63s23p6 |
| 19 | Potassium | 1s22s22p63s23p64s1 |
| 20 | Calcium | 1s22s22p63s23p64s2 |
| 21 | Scandium | 1s22s22p63s23p64s2 3d1 |
| 22 | Titanium | 1s22s22p63s23p64s2 3d2 |
| 23 | Vanadium | 1s22s22p63s23p64s2 3d3 |
| 24 | Chromium | 1s22s22p63s23p64s1 3d5 |
| 25 | Manganese | 1s22s22p63s23p64s2 3d5 |
| 26 | Iron | 1s22s22p63s23p64s2 3d6 |
| 27 | Cobalt | 1s22s22p63s23p64s2 3d7 |
| 28 | Nickel | 1s22s22p63s23p64s2 3d8 |
| 29 | Copper | 1s22s22p63s23p64s1 3d10 |
Shapes of Atomic orbitals of shells:
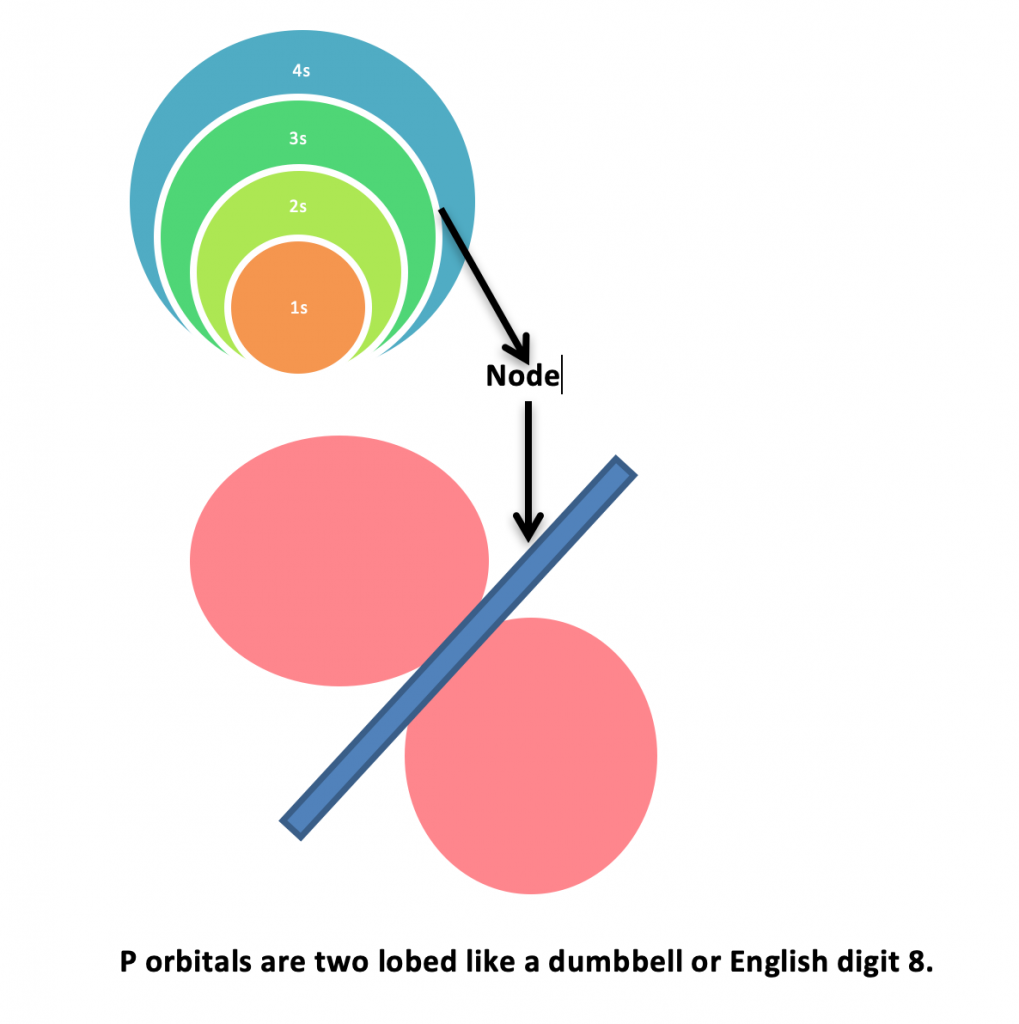
MainlyShapes of Atomic orbitals of shells include “s” Orbitals and “p” orbitals .
s orbitals are spherical in shapes. They are concentric which means one orbital in to other. Node is the space between two s orbitals where electrons probability is zero. Just for understanding, the white circular space between spheres is node.
p orbitals have two lobes dumbbell shape or English number 8 shape. The electrons probability is only with in the space of lobes because Nodes do not have any electron probability.
Orbital diagram
| Nitrogen | 1s2 2s2 2p3 |
So by now you have learned 2.2 The electron configuration of atoms.
You can read chemistry IA ideas here.
