Charle’s law of gases
Charles’ Law: Unlocking the Mechanics of Gases in Chemistry
Get a better understanding of gases in chemistry with Charles’ Law. Recommended for to grasp the concepts of physical chemistry, this guide will provide you invaluable insight into Charle’s law of gases.
Charles’ law of gases is a fundamental principle of physical chemistry which explains the behavior of gases under varying pressure and temperature. This guide will provide you with an overview of Charles’ law, from learning the basic definitions to understanding how its equations are used to solve problems in real-world scenarios.
Introduction to Charles’ Law
Charles’ law of gases is a gas law that determines the relationship between pressure, temperature and volume in an ideal gas. It was formulated by Jacques Charles, a French physicist and balloonist, in 1787. According to his law, as the pressure or temperature of an ideal gas increases, its volume also increases. Also known as the “law of volumes,” this relationship is expressed mathematically as follows: V1/T1 = V2/T2 where V1 and T1 are one set of pressure-temperature values while V2 and T2 are another.
Key Concepts of the Law
Charles’ Law provides an insight into the behavior of gases in chemical reactions, showing how different variables interact with each other. The key concepts of this law include Boyle’s law, which states that the volume of a confined gas is inversely proportional to its pressure, the ideal gas law which states that the pressure and temperature of a gas directly affect its volume and Dalton’s law, which states that the total pressure exerted by a mixture of gases is equal to the sum of pressures exerted by each individual gas.
The Equation and Calculations Related to It
Charles’ Law is given by the equation V/T = k, where V is volume, T is temperature and k is a constant. To solve for the volume of a gas under given conditions, we can rearrange the equation to V = kT. To obtain accurate values, both temperature values must be in Kelvin. Some chemists also multiply the value of k by an experimentally determined value to ensure more precise results.
Understanding Volume-Temperature Graphs
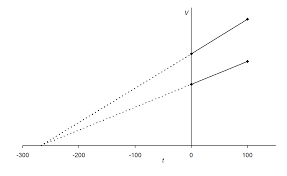
Charles’ law can also be used to illustrate the relationship between volume and temperature of a gas using a graph. As temperature increases, the volume of a fixed amount of gas also increases, and when plotted on a graph, will form an upward-sloping line. By plotting known values onto a Volume-Temperature (or V-T) graph, one can quickly interpret the required value from any given set of conditions.
Case Studies Representing Charles’ Law in Real World Applications
Charles’ law has a number of applications in the real world. For example, household furnaces make use of this law to ensure that they remain at a consistent temperature. As air continues to be heated up and moves away from the furnace, the increased volume results in decreased pressure. This causes cold air around the furnace to be drawn into it, helping to maintain a consistent temperature delivered by the furnace.
According to Charle’s law of gases “ the volume of a gas is directly proportional to the temperature at fixed pressure.”
V ∝ T ( T is in Kelvin)
Thus V/T is constant.
So if the Temperature of a gas changes from T1 to T2 , its volume will also change from V1 to V2.
Hence
V1/ T1= V2/ T2
Read About Boyle’s Law
