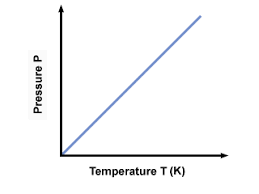
Learn about Gay Lussac’s Law : According to Gay Lussac’s Law “ the pressure of a gas is directly proportional to the temperature if the volume is fixed.”
Gay Lussac’s law is a basic law of chemistry that states the pressure of a given amount of gas is directly proportional to the temperature. This concept forms an important part of many scientific theories and experiments, and understanding it can help you make sense of much more complex topics related to gas laws.
What Is Gay Lussac’s Law?
Gay Lussac’s law states that for a given quantity of gas, the pressure it exerts is directly proportional to its absolute temperature. In other words, when the temperature increases, so does the pressure. This law is an essential part of modern chemistry and forms an important part in many scientific theories and experiments related to gas laws.
How Mathematical Relationships Help Describe Gay Lussac’s Law
In order to accurately describe Gay Lussac’s law, it is necessary to understand some basic mathematical relationships such as the ratio of pressure and temperature. For example, if Pressure P1 is equal to Temperature T1 and Pressure P2 is equal to Temperature T2, then the difference in pressure divided by the corresponding difference in temperature must always be a constant. This allows scientists and researchers to make accurate predictions about how the behavior of a gas may change when exposed to different conditions.
What Are the Effects of Pressure on Temperature?
Gay Lussac’s law states that when a gas is under pressure, its temperature also increases linearly. This means that if the pressure on a system is doubled, then the temperature of the system will also double. Increasing pressure can cause an increase in temperature and vice versa, as long as there are no outside influences changing either variable. In order to maintain an equilibrium, especially when studying gases within closed containers, it is important for researchers to consider this relationship between temperature and pressure.
How Does Ideal Gas Behavior Factor into Gay Lussac’s Law?
Gay Lussac’s law is applicable to ideal gases, which occupy an ideal container with perfectly uniform pressure and temperature. When a gas is assumed to be an ideal gas, this law can predict its behavior as pressure and volume of the gas change. Additionally, when dealing with non-ideal gases, one must consider the effects of heat capacity, which can cause small errors in predicting the temperature changes within a closed container when pressure is applied.
Analyzing Gases at Different Temperatures: The Adiabatic Process
The adiabatic process occurs when the gas is heated or cooled isothermally, and the pressure and temperature of the gas changes accordingly. To analyze this process, Gay Lussac’s law states that for any given mass of an ideal gas at a constant pressure, the change in temperature is directly proportional to its change in volume. This means that as one variable increases, so does the other. This relationship can be expressed through the equation PαVγT, where P is pressure, V is volume, and T is temperature.
P ∝ T ( T is in Kelvin)
Thus P/T is constant.
So if the Temperature of a gas changes from T1 to T2 , its volume will also change from P1 to P2.
Hence
P1/ T1= P2/ T2
Read About Boyle’s Law
Please share your thoughts on the content of Learn about Gay Lussac’s Law.
