Exploring Boyle’s Law of Gases and How It Works: If you want to understand the behavior of gases, Boyle’s Law of Gases is key. This law states that the pressure and volume of a gas are inversely proportional, meaning as one increases, the other decreases. In this guide, we’ll explore how this law works and what it tells us about gases.
What is Boyle’s Law?
Boyle’s Law of Gases states that the pressure and volume of a gas are inversely proportional. This means that if one increases, the other decreases. Boyle’s Law relies on Charles’s Law, which states that the volume of a gas is directly proportional to its temperature. This law helps explain how gases behave under certain conditions and can be used to calculate the pressure of a gas at different temperatures.
What Parameters Determine Gas Pressure?
Gas pressure is determined by several parameters, including atmospheric pressure, the amount of gas present and the temperature of the gas. At higher temperatures, gases take up larger amounts of space and become less dense, creating more pressure. On the other hand, at colder temperatures, gases become denser and exert less pressure. Boyle’s Law can be used to predict the changes in pressure when one or more of these parameters are changed.
How Does Gas Volume Impact Boyle’s Law?
Boyle’s Law states that as the volume of a contained gas increases, its pressure decreases. This occurs because the gas molecules have more space to move around in a larger container, which reduces their kinetic energy and causes them to exert less force against the walls of the container. Therefore, changing the volume of gas will cause the pressure inside the container to change accordingly.
How Does Temperature Affect Boyle’s Law?
Temperature plays an important role in Boyle’s Law. As the temperature increases, the amount of kinetic energy within a gas increases, causing its molecules to move faster and exert more force against the walls of its container. This increase in pressure causes the volume of the gas to decrease, creating an inverse relationship between temperature and volume. Thus, Boyle’s Law can be used to predict how changing the temperature of a gas will affect its pressure and volume.
Exploring Generalizations of Boyle’s Law
In addition to the temperature-pressure-volume relationship, Boyle’s Law can also be generalized to encompass additional factors such as the number of moles, or amount of material, contained in a volume of gas. This generalization states that the pressure of a gas is proportional to the product of its temperature and molar concentration. Thus, Boyle’s Law provides a fundamental basis for understanding how changing either of these two factors affects a gas’ pressure and volume.
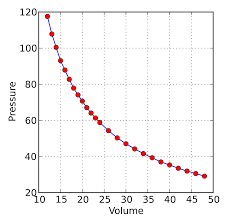
According to Boyle’s Law of gases , the volume of a gas is inversely proportional to the pressure of the gas at fixed temperature.
V ∝ 1/P
V=k/P
So k=PV
So if the pressure of a gas changes from P1 to P2 , its volume will also change from V1 to V2.
Hence P1 V1= P2V2
Read about Avogadro’s Law
