What are Atoms and Molecules?
Atoms and Molecules topic includes laws of chemical combination
Basic Laws of Chemical Combination
Antoine Lavoisier who is one of the founding chemist and Joseph Proust established the two basic laws of matter. These laws explain about the chemical combination of matter.
- Law of conservation of mass: According to this law mass can neither be created nor destroyed in a chemical reaction.
Foe example
If magnesium ribbon is burned in oxygen or air, the mass of reactants and products will not change.
2Mg ( 48g)+O2 (32g) = 2MgO(80g)
Mass of Mg 24×2=48g, O2 = 16×2=32g
It means in a chemical reaction the mass remains same.
Mass of reactants = Mass of products
2. Law of definite proportions: According to this law Atoms combine in definite ratio thus compound have definite proportion of atoms irrespective of the source of the compound.
For example Water,H2O has 11.11% Hydrogen no matter what ever is the source of water.
In other words, hydrogen and oxygen has mass in the ratio of 1 : 8 irrespective the source of water.
What is an Atom?
An Atom is the smallest particle of an element which can take part in a chemical reaction to form a new substance.
Atomic size or atomic radius : Atom is very tiny which cannot be seen by naked eyes. However the size can be measured in nanometer.
1nm=1/ 109 m
Atomic radii of hydrogen atom = 1 × 10–10 m.
Symbols of Elements: Dalton proposed some symbols for elements.
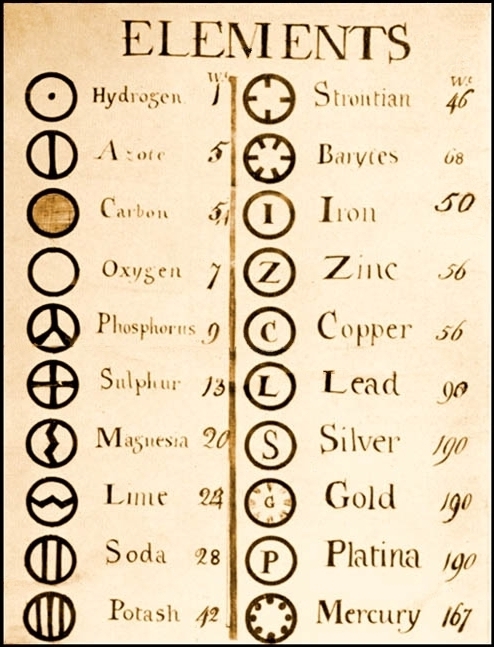
Modern day symbols of elements: IUPAC((International Union of Pure and Applied Chemistry) approves the names and symbols of elements. The first letter in capital of the name is generally used as a symbol however many elements also have second letter as well in the name. The second letter is always small letter.
For example: Berelium is Be and not BE.
Symbols of first 20 elements are as follows.
| Element | Symbol of element | |
| Hydrogen | H | |
| Helium | He | |
| Lithium | Li | |
| Berelium | Be | |
| Boron | B | |
| Carbon | C | |
| Nitrogen | N | |
| Oxygen | O | |
| Barium | Ba | |
| Fluorine | F | |
| Neon | Ne | |
| Sodium | Na | |
| Magnesium | Mg | |
| Aluminium | Al | |
| Silicon | Si | |
| Phosphorus | P | |
| Chlorine | Cl | |
| Argon | Ar | |
| Potassium | K | |
| Calcium | Ca |
What is a Molecule?
A molecule is the independent unit of a substance.
Types of molecules
Based on elements nature,Molecules are of two types:
- Molecule of an element: Such molecule consist only one type of atoms.
For example:
Oxygen gas molecule= O2
Hydrogen gas molecule= H2
Ozon gas molecule= O3
2. Molecule of compound: Such molecule consists of more than one type of atom.
For example:
Water molecule = H2O
Carbon di oxide molecule: CO2
Atomicity of molecule :Based on number of atoms, Molecules are of following types:
- Monoatomic molecule: All noble gases are monoatomic molecules because they do not combine with other atoms. They are all stable lements. They have complete outermost shell means octet except helium which has complete duplet( two electron)
- Diatomic molecule: Such molecules have two atoms in a molecule. These elements complete their outermost shell by combining between two atoms.
Example: Hydrogen molecule H2 – Single bond
Oxygen molecule O2 – Double bond
Nitrogen molecule N2– Triple bond
3. Tetratomic molecule: Such molecule has four atoms.
Example: Phosphorus molecule – P4
4. Polyatomic molecule: Such molecule contains many atoms.
Example: Sulphur molecule- S8. Sulphur molecule has eight atoms in one molecule.
What are ions?
Atoms tend to achieve stable configuration by either loss of electron or gain of electron. If an atom loses electron, it becomes a positive ion because the number of positive particles (proton) increases and the number of negative particles(electron) decreases.
Metals loose electrons to become positive ions. Positive ions are also called as cations.
Na( 11e and 11 p) – 1e à Na+(10 e and 11 p)
Similarly If an atom gains electron, it becomes a negative ion because the number of positive particles(proton) decreases and the number of negative particles(electron) increases.
Cl( 17e and 17 p) +1e à Cl–(18 e and 17 p)
What are polyatomic ions?
Polyatomic ions are the ions which are made up of two or more types of atoms.
Example:
Ammonium ion (NH4)+
Sulphite (SO3 )2-
Sulphate (SO4 )2-
Carbonate (CO3 )2-
Hydrogencarbonate (HCO3 )1-
Nitrate (NO3 )–
Nitrite (NO2 )–
Phosphate (PO4 )3-
Hydroxide OH–
Acetate CH3COO–
What is a Valency of an atom?
It is the number of electron lost or gained or shared by an atom in a chemical bond.
In general you can find valence of an atom by looking it in periodic table:
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Valency of transition elements is always variable so it is different in different compound.
Example:
Fe has its valency 2 in FeCl2 and it has valency 3 in FeCl3
How to write a Chemical Formula from the name of a compound?
Always Put metal or positive ion first in the formula.
Use the valency or charge and criss cross them.

What is Relative Atomic Mass(RAM)?
It is the mass of an atom compared with 1/12th part of c-12 isotope. Since it is relative to carbon or compared with carbon, it is not having any units.
All the periodic table mass are relative atomic mass.
What is relative molecular mass(RMM)?
It is the some of all the relative atomic mass of all the atoms in a molecule.
H2O = [(H = 1*2=2)+ (0 = 16) = 18]
What is Molar mass?
Mass of one mole of a substance is molar mass. It is always in grams. The Relative mass if used along with grams, it becomes Molar mass.
For example : RMM of water is 18. If we use gram with it, it will represent 1 mole water and it will be called as molar mass.
1 mole water= 18 g = molar mass. Thus molar mass unit is g/mole.
H2O = 18]
Similarly CO2= 44 g= Molar mass.
CO2= 12+16*2 = 44
What is Formula Unit Mass?
The ionic compounds are not considered as molecules instead as formula units.
The mass of formula unit of an ionic compound is also calculated in the same way by adding all the atomic mass.
For example KCl = 1 × 39 + 1 × 35.5 = 74.5
Read about Mole concept

