What is a Chemical bond? How is Ionic bond formed?
The force of attraction between two or more bonded atoms is
called chemical bond.
There are three types of chemical bonds:
What is duplet rule ?
The atoms try to become stable by acquiring the electronic structure of the nearest noble gas. When atoms try to acquire He configuration, it is called the duplet rule. Except for Helium, all the noble gases have eight electrons in their outer most shell so atoms try to get eight electrons in the outermost shell. This is called the Octet rule.
Atoms become stable by : Lose of outermost electrons( Metals become stable by lose of outermost electrons)
Gain of electrons( Non-Metals become stable by gain of electrons) or
Sharing of electrons(Non-Metals become stable by sharing of electrons)
The atoms near to He try to acquire 2 electrons in their outermost shell to become stable. It is called duplet rule.
Definition:
The electrostatic force of attraction between two or more oppositely charged ions is called ionic bond.
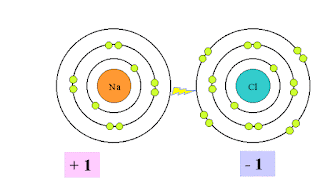
Example: The electrostatic force of attraction between Sodium ions (Na+) and chloride ions( Cl–) forms an ionic bond.
Metals loose valence electrons to become positive ions
( cations) because cations have more protons than electrons. Na atom has 11 p and 11 e but after lose of 1 e it left with 10 e but 11 p so 1 p is extra means 1 positive charge is extra so it becomes Na.+
Na – 1e àNa+
2,8,1 2,8
Non-Metals gain electrons to become negative ions( anions) because anions have less protons than electrons. Cl atom has 17 p and 17 e but after gain of 1 e it has with 18 e but 17 p so 1 p is less means 1 positive charge is less so it becomes Cl– .
Cl +1e à Cl–
2,8,7 2,8,8
Name of the ionic compounds
The cation is the first name
The anion is the second name. The anions generally end with ide like chloride, oxide, nitride etc
The compound ions like phosphate, carbonate, nitrate etc end with ate.
Sulphate ion, SO42-
Phosphate ion, PO43-
Hydrogen carbonate or bicarbonate ionHCO3–
Ethanoate (acetate ion), CH3CO2–
Hydroxide ion, OH–
Nitrate, NO3–
Carbonate, CO32-
Oxalate ion C2O4–
Ammonium ion is a positive ion , NH4+
Write the names of the following compounds
Na2O , NaOH, MgO, Mg(OH)2, H2O, Li2O
LiCl , MgCl2, P4O10, SO2, SiO2, Al2O3 , Al2(SO4)3
Physical properties of ionic compounds
1. High Melting and boiling point- large amount of energy is needed to break the strong electrostatic force of attraction between oppositely charged ions.
2. Crystalline in name – They form crystal lattice ( Large 3D network of ions)
3. Brittle- They breaks easily because of disturbance in the arrangement of ions.
4. Good conductor of electricity in aqueous solutions because of free movement of ions but bad conductor in solid state.

5. Soluble in water because the ions are separated and surrounded by water molecules.
We hope you enjoyed the article and found it informative. It would mean so much if you could take a moment to like, comment, and share this post with your network. Your support goes a long way in helping us create more content for you. Thank you for investing your time into reading this blog post!