What are Primary secondary and tertiary Carbon?
A Guide to Primary, Secondary, and Tertiary Carbon Atoms
Get a better understanding of the structure of organic molecules by breaking down primary, secondary, and tertiary carbon atoms. Check out our simple guide today!
Understanding the structure of organic molecules is crucial for understanding how compounds interact and react in chemical processes. One way to gain this understanding is through examining primary, secondary, and tertiary carbon atoms. This guide will help you understand the differences between these three types of carbon atoms.
What Is a Carbon Atom?
A carbon atom is an atom that consists of six protons and six electrons, with the electrons arranged in three pairs. Carbon atoms are the most important building blocks in organic chemistry, as they form strong bonds with other atoms and can be used to create complex molecular structures. Carbon forms single covalent bonds with its four valence electrons, allowing it to form compounds of different shapes and sizes.
The Different Types of Carbon Atoms.
In organic chemistry, there are three different types of carbon atoms – primary, secondary, and tertiary. Primary carbon atoms are connected to one other atom and have only one possible location for a hydrogen atom. Secondary carbon atoms are connected to two other atoms and have two possible locations for hydrogens. Lastly, tertiary carbons are connected to three other atoms and have three available sites for hydrogens. Each of these different types of carbons can create distinct structures within an organic molecule.
How Do Primary, Secondary, and Tertiary Carbons Affect Molecular Structure?
The types of carbon atoms found in organic molecules can greatly affect its overall structure and properties. Primary carbons tend to be fairly linear – meaning that the atoms connected to it will typically be directly in line with each other. Secondary carbons can form more interesting structures depending on the substituents attached – they may form straight chains, zigzag structures, or even bent ones. Tertiary carbons are not constrained by stereochemistry, creating a wide variety of distinct branching and cycling patterns.
How Does Molecular Geometry Differ Between the Three Types of Carbon Atoms?
Depending on the substituents attached to a particular carbon atom, its molecular geometry can be drastically different. Primary carbons are typically linear in structure with two atoms directly connected; secondary carbons will often form more interesting structures such as bent or zigzag shapes; tertiary carbons create branching or cycling patterns, allowing for more varied and complex molecule architectures.
Understand the Basics of Organic Chemistry by Knowing Your Carbons!
Carbon atoms are the fundamental building blocks of organic chemistry. By understanding the difference between primary, secondary, and tertiary carbons, you can better comprehend just how intricate and diverse molecule structures can be. Primary carbons have two hydrogen atoms or a single substituent attached; secondary carbons have three substituents forming an angle; tertiary carbon atoms have four different substituents forming a cyclic structure. Mastering this knowledge can give you a big boost in all types of organic chemistry!
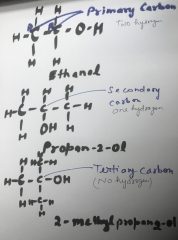
Primary Carbon:
The carbon atom which is attached to minimum two hydrogen atoms. If any functional group links to primary carbon than such compounds are known as primary compounds.
For example: Ethanol, C2H5OH, is a primary alcohol.
Secondary Carbon:
The carbon atom which is attached to only one hydrogen atom. If any functional group links to secondary carbon than such compounds are known as secondary compounds.
For example: Propan-2-ol, CH3CH(OH)CH3, is a secondary alcohol.
Tertiary Carbon:
The carbon atom which is not attached to any hydrogen atom. If any functional group links to tertiary carbon than such compounds are known as tertiary compounds.
For example: 2-methylpropan-2-ol, C(CH3)3OH
Motivation 2.0 : “THE ULTIMATE STUDENT SURVIVAL GUIDE”