Aufbau Principle and filling of electrons in orbitals and sub shells
The Aufbau Principle is a concept used to explain the electronic configuration of atoms. The principle states that electrons occupy the lowest available energy levels in an atom, from lowest to highest energy. While no single scientist is credited with discovering this principle, it has been referenced by many scientists over time and continues to be fundamental in understanding atomic structure. Aufbau Principle for electronic configuration is not on the name of any scientist as usually happened in sciences. Aufbau means building up.
Electrons are build up in orbitals of different subshells of different energy levels on the basis of energy.
Electrons first fill lower energy level and then move to next higher level.
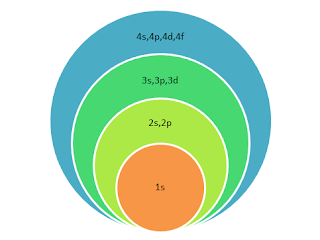
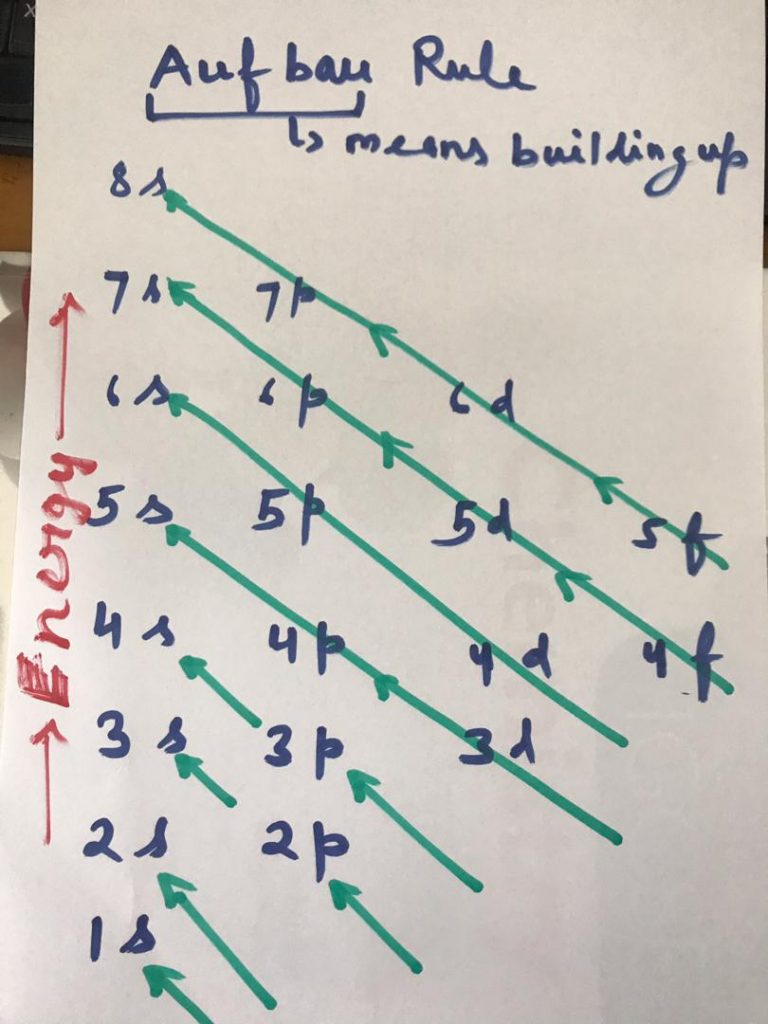
So electrons start filling from 1s orbital on wards in the following sequence:
1s,2s,2p , 3s,3p,4s,3d,4p,5s,4d,5p,6s,4f,5d,6p,7s,5f,6d,7p,8s
Simply it can be learned as ssp sps dps dps fdps fdps

| Atomic Number | Element | Electronic Configuration |
| 1 | Hydrogen | Is1 |
| 2 | Helium | Is2 |
| 3 | Lithium | 1s2 2s1 |
| 4 | Beryllium | 1s2 2s2 |
| 5 | Boron | 1s2 2s2 2p1 |
| 6 | Carbon | 1s2 2s2 2p2 |
| 7 | Nitrogen | 1s2 2s2 2p3 |
| 8 | Oxygen | 1s2 2s2 2p4 |
| 9 | Fluorine | 1s2 2s2 2p5 |
| 10 | Neon | 1s2 2s2 2p6 |
| 11 | Sodium | 1s22s22p63s1 |
| 12 | Magnesium | 1s22s22p63s2 |
| 13 | Aluminium | 1s22s22p63s23p1 |
| 14 | Silicon | 1s22s22p63s23p2 |
| 15 | Phosphorus | 1s22s22p63s23p3 |
| 16 | Sulphur | 1s22s22p63s23p4 |
| 17 | Chlorine | 1s22s22p63s23p5 |
| 18 | Argon | 1s22s22p63s23p6 |
| 19 | Potassium | 1s22s22p63s23p64s1 |
| 20 | Calcium | 1s22s22p63s23p64s2 |
| 21 | Scandium | 1s22s22p63s23p64s2 3d1 |
| 22 | Titanium | 1s22s22p63s23p64s2 3d2 |
| 23 | Vanadium | 1s22s22p63s23p64s2 3d3 |
| 24 | Chromium | 1s22s22p63s23p64s1 3d5 |
| 25 | Manganese | 1s22s22p63s23p64s2 3d5 |
| 26 | Iron | 1s22s22p63s23p64s2 3d6 |
| 27 | Cobalt | 1s22s22p63s23p64s2 3d7 |
| 28 | Nickel | 1s22s22p63s23p64s2 3d8 |
| 29 | Copper | 1s22s22p63s23p64s1 3d10 |


