Exploring the Shapes of Atomic Orbitals of Shells
Exploring the Shapes of Atomic Orbitals of Shells Mainly Shapes of Atomic orbitals of shells include “s” Orbitals and “p” orbitals .
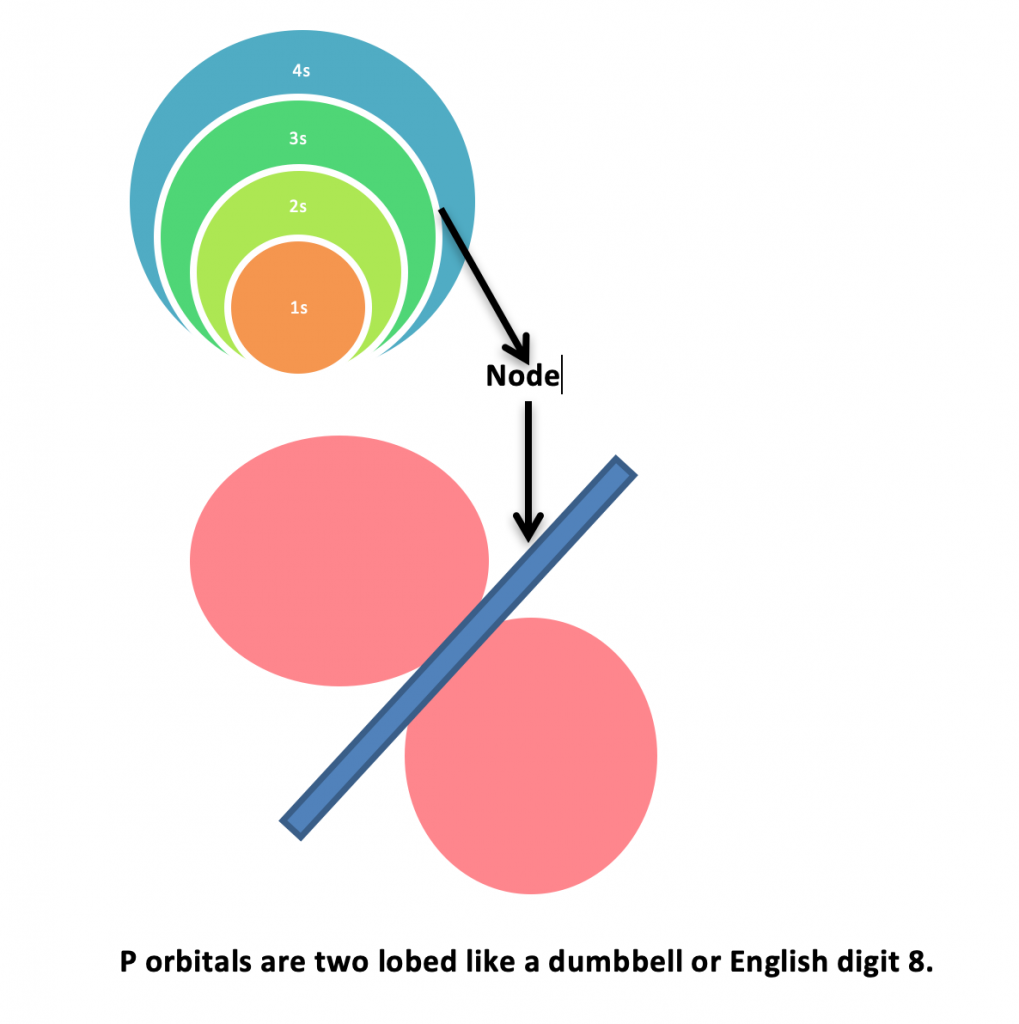
s orbitals are spherical in shapes. They are concentric which means one orbital in to other. Node is the space between two s orbitals where electrons probability is zero. Just for understanding, the white circular space between spheres is node.
p orbitals have two lobes dumbbell shape or English number 8 shape. The electrons probability is only with in the space of lobes because Nodes do not have any electron probability.
At the heart of quantum mechanics is a concept known as atomic orbitals—specific shapes that govern the behavior of electrons inside atoms. An atomic orbital describes the probability of an electron being in a certain area around the nucleus, and they can be used to explain why an atom behaves as it does in different situations.
What are Atomic Orbitals?
Atomic orbitals are mathematical functions that describe the probability of an electron being in a certain area around the nucleus of an atom. The shapes of these orbitals change according to a set of quantum numbers, which can be used to describe the amount and type of energy in the system. In general, each shell has a distinct shape—the s-orbital is spherically symmetrical, while all others are more complex and may vary depending on the particular atom or ion. By understanding these shapes, scientists can better understand how electrons behave inside atoms and how different elements bond with each other.
The Six Quantum Numbers Describing Atomic Orbitals
Each atomic orbital is determined by six quantum numbers: the principal quantum number (n), the angular momentum quantum number (l), the azimuthal quantum number (ml), the magnetic quantum number (ms), and the spin quantum numbers for both electrons (s). Together, these six numbers uniquely define each atomic orbital, providing information about its energy, shape and orientation in relation to the other orbitals.
Electron Configurations and the Shapes of Shells
Electron configurations refer to the arrangement of electrons in an atom. This arrangement is determined by the shells and subshells that encompass the electrons, which are shaped differently depending on the electron’s quantum numbers. The shapes of these shells or orbitals vary: some are spherical with equal radii (s orbitals), while others appear as dumbbell shapes or two overlapping lobes (p orbitals). These shapes can be very helpful when understanding how atoms interact with each other.
Bonding: Putting It All Together
When it comes to bonding, looking at the type and number of orbitals that compose a shell can help explain why some atoms have stronger bonds than others. This is because depending on their shapes and energies, orbitals may experience repulsion or attraction when interacting with one another. For example, when two p orbitals overlap, a strong pi bond is formed that is three times stronger than an s-s bond. Knowing about the shape of an atom’s electrons helps to elucidate how these bonds work in different molecules.
The Effects of Physical Environment on Atomic Orbitals
Atomic orbitals are affected by the physical environment of a particular atom. For example, when two or more atoms interact, their electron clouds will interfere with each other and this can change the shapes of their atomic orbitals. Electron repulsion also plays a role in altering the energy level of an orbital and its spatial orientation. Ultimately, understanding where and how these shells form allows us to explain the quantum mechanical behavior of different elements.
Read Quantum mechanical model of Atom

