How Does the Law of Conservation Of Mass Work?
What is the law of conservation of mass and what do we need to know about it? Check out this comprehensive guide that covers everything from fundamentals to applications.
Article
The law of conservation of mass is an important principle in physics, chemistry and biology that states that energy and matter can be neither created nor destroyed. This means that the total amount of mass within a closed system remains constant. Learn more about this law, including its fundamentals, history and applications.
Understand the Fundamental Concept of Mass Conservation.
The basic concept of the law of conservation of mass is that the total amount of mass in an isolated system remains constant, no matter what changes occur within the system. This idea is rooted in ancient beliefs in the Greek atomists and was further developed by the English naturalist John Dalton during the 19th century. The law states that matter can neither be created nor destroyed, which means that everything on earth is composed of limited resources.
Learn about Matter and Energy Transformations.
To better understand the law of conservation of mass, it’s important to learn about matter and energy transformations. As different forms of energy are transformed from one form to another, the total amount of mass remains unchanged. For example, energy can be converted from chemical energy stored in a battery into electrical energy by the battery. The electrical energy can then generate heat, light, or sound waves—but the total amount of mass remains unchanged.
Dig into Examples of Mass Conservation.
When it comes to the law of conservation of mass, you can better understand its implications by looking at examples. Here are a few scenarios you may come across when studying the law of conservation of mass: Chemical reactions in which substances react to form new ones with different components but no change in total mass; Nuclear reactions such as fission and fusion that transform mass into energy, leaving behind residue particles with no net change in overall mass; And the transformations of matter between solid, liquid and gas states, where matter is neither created nor destroyed— it just changes forms.
Examine The Modern Interpretation of the Law of Conservation of Mass.
The modern interpretation of the law of conservation of mass states that mass can be neither created nor destroyed— it can only change form. This means that while substances may undergo chemical, physical or nuclear reactions with different components and results, there is no net change in total mass in these processes. In addition, energy may appear or disappear in a closed system, but the total quantity of energy remains constant. This is known as the law of conservation of energy.
Discover How to Use The Law in Everyday Life and In Science Experiments.
Understanding the law of conservation of mass is important for both science experiments and everyday life. The most common way this law is utilized in everyday situations is through energy conservation— ensuring that energy use does not exceed available resources. In science, this law is used to prove or predict outcomes within a system. For example, if one knows the reactants in a chemical reaction, this law can be used to predict the expected amount of products after the reaction concludes.
Matter can neither be created nor destroyed which is clear by Law of conservation of mass examples
2Mg ( 48g)+O2 (32g) = 2MgO(80g)
Mass of Mg 24×2=48g, O2 = 16×2=32g
It means in a chemical reaction the mass remains same.
CH4 (16g) + 2O2 (64g) = CO2 (44g) + 2H2O (36g)
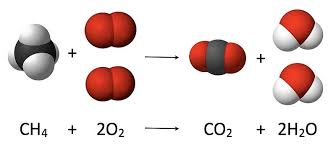
Read Law of definite proportions
How do you find the content in the post “How Does the Law of Conservation Of Mass Work?”
