An Overview of Lattice Enthalpy – Chemical Reactions & Compounds
Get to know what lattice enthalpy is and how it affects chemical reactions & compounds. This concise overview provides a broad introduction to the concepts behind lattice enthalpy.
Lattice enthalpy is an important concept in chemistry, providing a measure of the energy needed to break apart a crystal lattice and form isolated molecules or ions. This conceptual overview gives a brief explanation of this chemical concept and how it affects compound stability and chemical reactions.
What is Lattice Enthalpy?
Lattice enthalpy is a measure of the energy needed to separate particles in an ionic crystal lattice. It is defined as the amount of energy released when one mole of a solid ionic compound is formed from its constituent gaseous ions. In other words, it provides us with a measure of how strong or stable the chemical bonds are between atoms or ions in an ionic compound.
Factors Influencing Lattice Enthalpy.
There are a few key factors that influence how strong or stable the ions in an ionic compound are and thus affect lattice enthalpy. These include the charge on the ions, the sizes of the ions, and the distances between them. The greater the charge on an ion, the stronger its attraction will be toward other opposite-charge ions, leading to a high lattice enthalpy. Similarly, if two ions of small charged sizes are close together they can form strong bonds and result in higher lattice enthalpies.
Trends in Lattice Enthalpy & How It Affects Compounds and Reactions.
The value of lattice enthalpy for a given compound can be studied in terms of trends by looking at the differences between various types of compounds as elements move along rows and columns on the periodic table. Generally, as you go down a group, the size of an ion increases, leading to weaker attractions between ions and thus lower lattice enthalpies. Similarly, when going to the right across a period, the charge on an ion increases, so there are more attractive forces leading to higher lattice enthalpies. Understanding these trends is important because lattice enthalpy affects numerous properties associated with a compound such as its melting point and solubility.
Understanding the Significance of Lattice Enthalpy In Chemistry.
Understanding the value of lattice enthalpy and how it affects chemical reactions is essential for chemists in many fields. In general, a high lattice enthalpy suggests the compound is more likely to be more stable. This means that higher energy must be used in order to break the molecular structure apart and form new molecules during a reaction. Similarly, compounds with low lattice enthalpies tend to react more easily, since less energy is required to create a reaction. Knowing this information can help scientists design better reactions and create applications for compounds with predetermined properties.
Using The Energy Changes To Predict Chemical Reaction Heat Outputs & Entropies
By measuring the change in energy when molecules undergo a reaction, we can use this data to predict the heat output of a chemical reaction. This is accomplished using the enthalpy of formation, which is the amount of energy it takes to form 1 mole of a compound from its constituent elements. Additionally, these changes can also be used to calculate entropy, which represents the disorganization within a system and quantifies how much energy is no longer available for useful work in the substance after a reaction has occurred.
It is the amount of energy required to break one mole of crystal lattice(ionic solid) into its constituent ions in gaseous state.
Since energy is needed to break the crystal lattice hence this reaction is endothermic reaction. for example MX(s) is an ionic solid and breaks down to its constituent ions (M+(g) and X–(g)). The energy needed to break MX(s) is lattice enthalpy.
MX(s) –>M+(g) + X–(g)
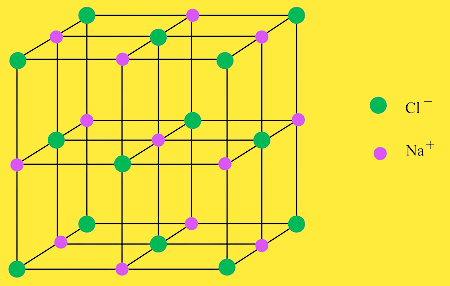
What is a crystal lattice?
It is the three dimensional network of cations( Positive) and anions(negative) in an ionic solid.
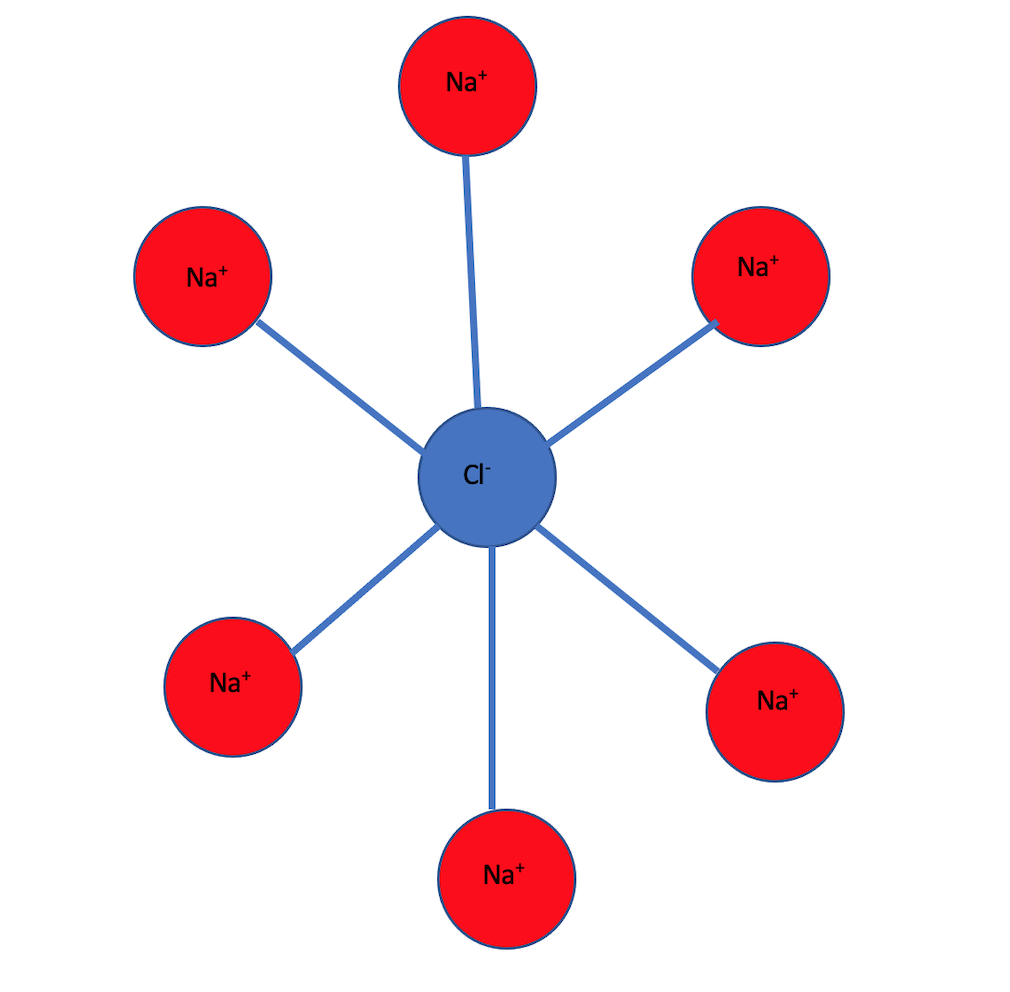
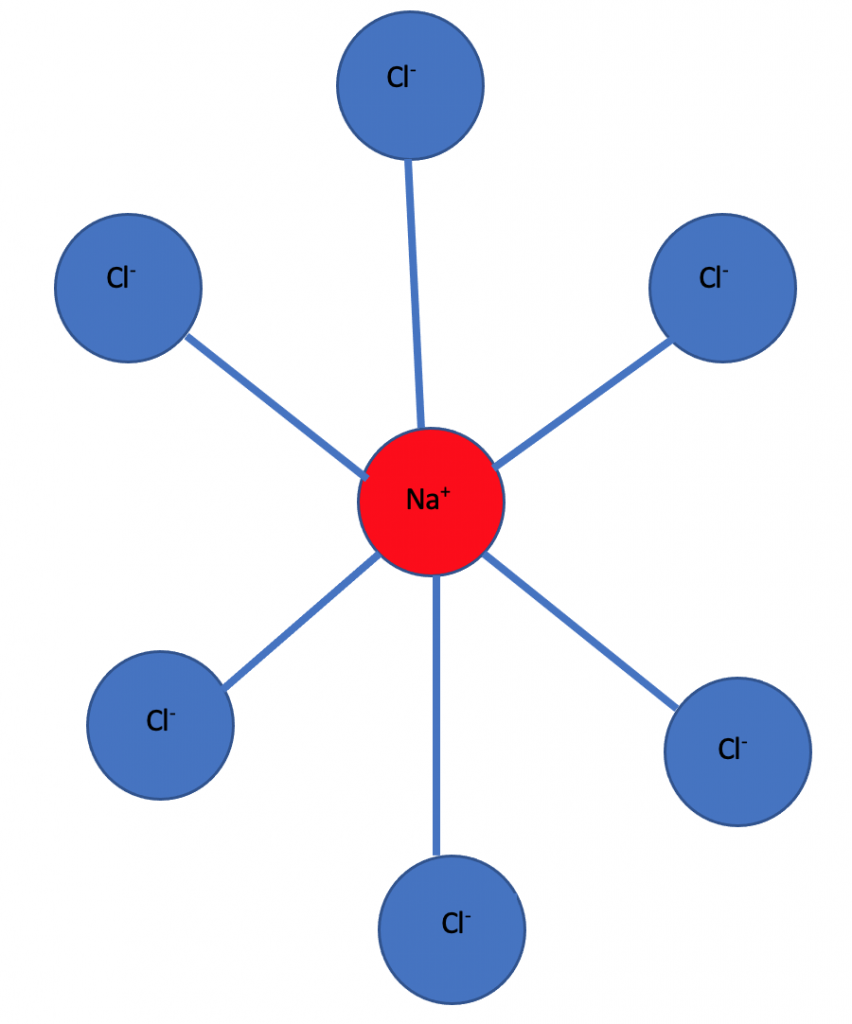
For example sodium chloride(NaCl) is an ionic solid and has a crystal structure in which each sodium ion is surrounded by six chloride ions and each chloride ion is surrounded by six sodium ions.
Hope you have found An Overview of Lattice Enthalpy useful in your learning.
